the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Paul J. Crutzen – interactions with friends and colleagues
Lennart Bengtsson
John Birks
John Burrows
Ruprecht Jaenicke
Richard S. Stolarski
Paul J. Crutzen was a brilliant scientist and a pioneer in atmospheric sciences. At the same time, he was a kind-hearted, humorous and pleasant person. Paul was deeply empathetic toward the personal lives of his colleagues and students, always making time for those around him – especially his family. This tribute brings together a series of anecdotes shared by friends and colleagues, offering a more intimate portrait of the man behind the science. Collectively, these reflections reveal aspects of Paul Crutzen that may be overlooked when focusing solely on his extraordinary scientific accomplishments; however, they were integral to his ability to achieve them.
- Article
(5912 KB) - Full-text XML
- BibTeX
- EndNote
Paul Crutzen was a brilliant scientist. Having begun his career as a bridge-building engineer, what makes his scientific achievements all the more remarkable is the breadth of topics to which he made significant contributions over the years (Crutzen, 1996; Müller, 2022; Fishman et al., 2023). His interests spanned topics relevant to a majority of the atmosphere, with a particular focus on the mesosphere, stratosphere and troposphere. Among other things, he first described the NOx-driven ozone-loss cycle in the stratosphere (Crutzen, 1970), he recognised that emissions of NOx caused by a possible fleet of supersonic planes could have a detrimental effect on stratospheric ozone (Crutzen, 1970; Johnston, 1971; Crutzen, 1972), he investigated how tropospheric nitrogen-containing compounds (like N2O) can enter the stratosphere and cause the formation of stratospheric NOx (Schütz et al., 1970; Crutzen, 1970; Crutzen and Ehhalt, 1977), and he was among the first to develop mechanisms for the chemical formation of ozone in the troposphere (Crutzen, 1973; Chameides and Walker, 1973; Fishman and Crutzen, 1978; Fishman et al., 1979a, b). He further contributed key ideas on how to explain the “ozone hole” (Crutzen and Arnold, 1986); today the focus is on the recovery (or “healing”) of the Antarctic ozone layer (e.g. Kuttippurath et al., 2013; Solomon et al., 2016; WMO, 2022). Paul also made fundamental discoveries on the impact of biomass burning and aerosol particles on the troposphere (e.g. Crutzen et al., 1979; Crutzen and Andreae, 1990; Lelieveld et al., 2001; Ramanathan et al., 2001).
Moreover, Paul's work on smoke from fires after a hypothetical nuclear war inspired new research on a concept now known as “nuclear winter” (e.g. Crutzen and Birks, 1982; Birks and Crutzen, 1983; Turco et al., 1983; Robock, 1984; Robock et al., 2023). By writing an essay (Crutzen, 2006), he also initiated the resumption of the discussion on “geoengineering”, a concept today referred to as “climate intervention” (Visioni et al., 2023). Paul coined the term “Anthropocene” (e.g. Crutzen, 2002; Crutzen and Steffen, 2003; Crutzen and Müller, 2019; Benner et al., 2021), and in 2000, he was also one of the founders of the journal Atmospheric Chemistry and Physics (Müller et al., 2023; Ervens et al., 2025), which pioneered transparent peer review and open access (Pöschl, 2012; Ervens et al., 2025). Initially, his research focused on understanding the fundamental processes of the Earth's atmospheric system. Over time, however, the driving force behind his scientific work shifted toward understanding the origins of human-induced impacts on the atmosphere and climate – as well as on finding ways to mitigate these effects (e.g. Crutzen, 1996; Müller, 2022; Fishman et al., 2023; Müller et al., 2023).
Beyond his scientific achievements, Paul played a pivotal role in advancing atmospheric and climate science through his mentoring and education of young researchers. Many outstanding scientists began their careers under his guidance. Several of them are still active today. Many of these scientists later became mentors themselves, guiding a new generation of researchers. As a result, Paul can now be considered to have “scientific grandchildren – and even great-grandchildren”.
Paul himself provided an excellent description of his life and of his scientific work in his Nobel Prize lecture (Crutzen, 1996) on the occasion of the 1995 Nobel Prize in Chemistry, which he shared with Mario J. Molina and F. Sherwood Rowland (Harris, 2020). Further, shorter biographical texts (Möllers et al., 2015; Lelieveld, 2021; Moortgat et al., 2021; Rodhe, 2021; Solomon, 2021; Zalasiewicz et al., 2021; Zetzsch, 2021) and more detailed memoirs (Lax, 2018; Müller, 2022; Müller et al., 2023; Fishman et al., 2023) are available. The idea of the present contribution is not to enhance previous biographical texts but, rather, to complement this information with personal accounts of an interaction with Paul by a number of his colleagues and friends. Paul had a profound influence on the scientists he worked with, thanks to his unique blend of scientific curiosity and deep humanity.
Paul Jozef Crutzen was born in Amsterdam on 3 December 1933. He remained a Dutch citizen all his life. He grew up with his younger sister Elisabeth (Lies) (Fig. 1), who spent her life in Amsterdam. Paul passed away in Mainz on 28 January 2021. On 14 February 1958, in Amsterdam, he married Terttu Soininen. This day is now known as “Valentines day”, but Paul and Terttu were not aware of this fact in 1958. Terttu and Paul have two daughters, Ilona and Sylvia (Fig. 2), and three grandchildren. Paul's life has been described in detail elsewhere (e.g. Lax, 2018; Müller, 2022; Fishman et al., 2023); thus, a description of his life is not repeated here.
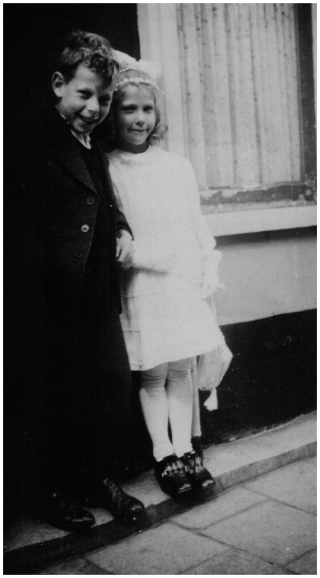
Figure 1Paul Crutzen as a child with his younger sister, Elisabeth (Lies). (Picture courtesy of Ilona Crutzen.)
On 1 July 1959, Terttu and Paul moved to Stockholm. Paul initially took a position at the Meteorological Institute of Stockholm to work in computing. However, his potential was quickly recognised, and alongside his duties he managed to complete his degree in meteorology. He then continued as a doctoral student at the institute, ultimately earning the Swedish equivalent of a doctorate. In 1973, he completed the “Filosofie doktor”, which is roughly equivalent to a habilitation (Müller, 2022). In 1974, along with his family, Paul moved to Boulder, Colorado (USA); he first took positions at both NOAA (National Oceanic and Atmospheric Administration) and NCAR (National Center for Atmospheric Research). Later, in 1977, he became a director at NCAR. In 1980, he moved back to Europe and accepted the position of director of the Air Chemistry Department at the Max Planck Institute for Chemistry (MPIC) in Mainz as the successor of Christian Junge. Ruprecht Jaenicke went to the University of Mainz at the same time. Ruprecht possesses a movie clip in which Paul explains that he proposed Christian Junge for the Nobel Prize. This shows how much Paul valued Christian Junge.
Paul was from the Netherlands, so Dutch was his native language. On the occasion of his visit to Jülich on 15 February 1996 (Sect. 3.6.2), Paul spoke in Dutch to a Dutch employee at the “Forschungszentrum Jülich” (Martin Böhmermann). Martin Böhmermann could hear clearly from Paul's accent that he was from Amsterdam – and (in Martin's words) not from the richest part of town.
Paul's parents on his mother's side were of mixed German and Polish origin and lived in the Ruhr region in Germany, while his father had relatives in the Netherlands, Germany and Belgium. Thus, at a young age, Paul inherited a cosmopolitan view of the world. He learned the value of being fluent in several languages, as his own family spoke a mixture of languages and dialects. At school, he learned French, English and German; however, later in Sweden, Swedish became the language of the Crutzen family (Müller, 2022). He had also started learning Finnish – Rolf Müller recalls that Paul translated the legend of a Finnish plot from Sodankylä for him. However (in Paul's own words), he stopped learning Finnish as this was part of an agreement with his wife: she would stop smoking (which she did very moderately, but which he hated nonetheless) and he would learn Finnish. However he pointed out that once he had learned Finnish, he could not unlearn it, whereas Terttu could start smoking again!
Despite his concentration on science, Paul was a very warm-hearted, friendly and humorous person, who always had time for his family (Fig. 2). He never forgot how important the private life of his colleagues and students was – a fact that was an intrinsic part of any collaboration or interaction with Paul (Müller, 2022; Müller et al., 2023; Fishman et al., 2023). Having set the scene, this contribution offers a collection of stories – both scientific and personal – shared by colleagues and friends who interacted with Paul.
3.1 Conversations with Lennart Bengtsson in summer 1995
Paul Crutzen contacted his good friend Lennart Bengtsson in summer 1995. He asked if Lennart could arrange for him to be invited to the Nobel Prize ceremonies later that year. Of course, Lennart knew things he could not talk about at that time, so he did not say anything. All that Lennart said was that he would work on the issue and that he was confident that things could be resolved eventually. Of course, things were resolved, and Paul was indeed invited to Stockholm towards the end of 1995 for reasons everybody knows today.
3.2 John Birks remembers
The first time that John Birks ever interacted with Paul was when they were on the same flight from Champaign, IL, to Washington, DC, and Paul arranged for them to sit together. The flight had an intermediate stop somewhere, and John realised that his bag had only been checked to that airport. Therefore, he had to get off the plane, collect the bag and recheck it. When he had not re-boarded and they were closing the door to the plane, Paul convinced the flight attendants to hold the plane for John.
John greatly admired Paul and got to know both Paul and Terttu very well during his year in Mainz. Paul was part of the reason that John moved from the University of Illinois to the University of Colorado when Paul was at NCAR, and he followed him to Mainz for his first sabbatical a year after Paul had moved to Mainz (Sect. 2). John lost touch with Paul after the nuclear winter days, but they joined forces again to work on asteroid impacts around the time that John retired from university and started his company.
3.3 John Burrows remembers
3.3.1 First introduction to Paul Crutzen
John Burrows first met Paul Crutzen in early March 1977 at the NATO winter school in Arabba, the Dolomites, Italy, when John was a doctoral student at the University of Cambridge (under the supervision of Brian Arthur Thrush). John was studying free-radical reactions of importance in the atmosphere. The meeting in 1977 was organised by Karl-Heinz Becker of the Bergische Universität Wuppertal. It brought together many of the leaders of the then rapidly evolving field of atmospheric chemistry – but also involved the younger generation in this field. One of the reviewers of this paper also attended the winter school in Arabba.
Paul gave some impressive lectures at the winter school, the second half of which were particularly remarkable, as he delivered them after sustaining a broken leg while skiing down the Marmolada in bad weather. Paul was a gifted communicator and filled the young and older scientists at this school with his enthusiasm about the importance of studying stratospheric and tropospheric ozone. This meeting demonstrated the fun that this senior scientist found in his research.
3.3.2 Joining Paul's department
While John Burrows was a researcher at the UKAEA (United Kingdom Atomic Energy Authority) and at the University of Oxford, he was offered a position at Paul's new Air Chemistry Department at the Max Planck Institute for Chemistry in Mainz (see Sect. 2), joining in late 1981 to work with his group leaders, Geert Moortgat and David Griffiths, on kinetics and spectroscopy of key atmospheric constituents. John was attracted by the lively atmosphere of this new department.
During this period, Paul, together with John Birks, was busy investigating the impact of a hypothetical nuclear war on the atmosphere (see Sect. 3.2). Their findings, which they called “twilight at noon” (Crutzen and Birks, 1982), later became known as nuclear winter (Robock et al., 2023). These studies and the controversy at that time led to fascinating coffee time discussions. Paul did an excellent impression of Edward Teller, the father of the H Bomb, who was at least initially sceptical about the concept of nuclear winter. Apparently Teller gave monologues bearing down on his audience without ever looking at any notes.
John is also grateful to Paul and Terttu, who regularly invited him, his future wife and other colleagues to dinner, where the after-dinner coffee from Terttu was a special treat. Paul and Terttu were both good at looking after John without him realising it. John Burrows also shared a general passion for sport with Paul, in particular football1 (Müller et al., 2023). Paul had played for Ajax Amsterdam youth teams in his youth and, at the age of 50, was still a good midfield player. He played in the Air Chemistry All-stars football team, which bound all players together. John also enjoyed watching international football games with Paul and Terttu. Although Paul always had a busy schedule, he found time for such activities.
3.3.3 Paul as a science manager
Paul was a unique person, a powerful advocate for research, full of ideas and always trying to push the envelope of our knowledge. He led by example. In managing people, he developed individual relationships with his scientists. He was able to encourage and inspire, while also being a kind, good-humoured and warm-hearted mentor. He played an important role in John Burrows remaining in research, particularly when John was unsure about his own future.
Paul was also a calculated risk-taker. In this respect, he should be seen as a “why not” person rather than a “why” person. In general, as well as having his own great ideas about atmospheric chemistry, he would listen to the ideas of others about science priorities carefully. He was always interested in hearing about potentially important and doable atmospheric science.
One personal example is as follows: in 1983, Paul asked John Burrows to move to his new research group called “Optical Measurements of Atmospheric Constituents”, joining Dieter Perner. The focus was to be on remote-sensing and in situ measurements of key atmospheric constituents. Over a coffee time discussion, Dieter, Paul and John talked about some potential remote-sensing options. They discussed the use of differential optical absorption spectroscopy (DOAS), which had been developed by Dieter and Ulrich Platt at Forschungszentrum Jülich (e.g. Perner and Platt, 1979). John thought that this idea could be exploited successfully when applied to space-borne spectrometer measurements. They both agreed! This led to Paul's and Dieter's support and, in 1984, to the first MPI attempt to propose a mission in response to an ESA (European Space Agency) call for ideas to exploit a free-flying small satellite for Earth observation, released from the space shuttle. This proposal failed, but it gained the ESA's attention. This then led to John leading the development of the SCIAMACHY (SCanning Imaging Absorption spectrometer for Atmospheric CHartographY) and SCIA-mini proposals, supported by Paul, Dieter and a strong team at the MPI in Mainz, as well an international science team (Burrows et al., 1995).
3.3.4 Paul as a government adviser and science advocate
Paul was someone who researched science of importance to society. In this context, Paul also served science by successfully advising government. Of particular relevance here was his participation in the Federal German Government Enquete Commission entitled “Precautionary measures to protect the Earth's atmosphere” from 1987 to 1991 (Schmidbauer, 1990). Paul also contributed significantly to the international activities for protecting the ozone layer (i.e. the Montreal Protocol and its amendments and adjustments; see WMO, 2022, and references therein).
Thanks to Paul, John had the pleasure of travelling with Paul and reporting on atmospheric remote-sensing opportunities for Germany at a formal meeting of this Commission at the Ministry for Science and Technology in Bonn. They had the Dornier company (now Airbus), as the prime industrial contractor on hand, demonstrate the technical feasibility of the proposed concepts.
In its broad-ranging report (Schmidbauer, 1990), the Enquete Commission of the German parliament made important statements about the value of remote sensing for atmospheric research. The German research ministry (BMFT), under Heinz Riesenhuber, used this advice and created its ATMOS programme, including support for SCIAMACHY (nationally) and the Earth Observation programme of the ESA (internationally). In 1986, West Germany alone was responsible for more than 10 % of the tropospherically long-lived ozone-depleting substances (Dickman, 1988), which were primarily chlorofluorocarbon (CFC) compounds and halons (WMO, 2022). In more detail, the global consumption of CFC-11 (1976–1986) was 300 ± 50 kt yr−1, while the consumption of CFC-12 (1976–1986) was 350±50 kt yr−1. In the European Union, the production of CFC-11 and CFC-12 together (1976–1986) amounted to 320±20 kt yr−1, while the EU production in 1986 was 204 kt of CFC-11 and 168 kt of CFC-12. In Germany, consumption (1980s; all fully halogenated CFCs) was 60–100 kt yr−1 (Schmidbauer, 1990, p. 181 ff.). Note that consumption and production figures are not comparable, as the latter also include exports.
The release of ozone-depleting substances and halons to the atmosphere resulted in a global depletion of the stratospheric ozone layer and the appearance of an “ozone hole” over Antarctica in the Southern Hemisphere springtime polar vortex, first reported on the basis of ground-based measurements by Farman et al. (1985). Consequently the SCIAMACHY and SCIA-mini proposals were timely.
3.3.5 Paul as an advocate for atmospheric remote sensing from space
Paul first exploited NASA (National Aeronautics and Space Administration) 4 BUV (Backscatter Ultraviolet) ozone measurements to show the impact of solar proton events and the production of oxides of nitrogen on stratospheric ozone (Solomon and Crutzen, 1981). He was a primary investigator and advocate for the NASA Halogen Occultation Experiment (HALOE) on the Upper Atmosphere Research Satellite (UARS), which was launched in September 1991 (Russell et al., 1993; Crutzen et al., 1995). He gave John the following advice for SCIAMACHY: one needs to be careful to keep space experiments well focused and to realistically manage expectations. He liked to call SCIAMACHY “sky magic”.
SCIAMACHY was proposed in July 1988 for the ESA polar platform, later renamed Envisat. In December 1988, SCIA-mini, which was subsequently descoped and renamed GOME (Global Ozone Monitoring Experiment) was selected by ESA for a fast-track launch on ERS-2 (Burrows et al., 1999). Paul coordinated the modellers, who supported the proposal and joined the science team. He was excellent, possibly unique, at facilitating science. Without Paul, it would not have been possible to win these game-changing space missions.
3.3.6 Paul as a mentor
Paul was a great mentor. He was modest by nature, and he once told John Burrows late in his life that he had learnt a great deal from George Witt and Bert Bolin, his supervisors at the University of Stockholm. He said that they had recognised some aptitude in him (Müller, 2022; Fishman et al., 2023) and then unselfishly facilitated his scientific research. This was how he later advised and guided the large number of scientists who worked with him. In early 1991, Paul came to John's office and said that he should apply for a vacant position at the University of Bremen, the advertisement for which Paul just had received. He said he thought that this was a good opportunity for John and would also provide the continuity for SCIAMACHY, as the University of Bremen wanted to build up its natural sciences. This initial discussion then led to John's later move to Bremen in 1992.
Paul remained a great support for the exploitation of space-based instrumentation for atmospheric science, and John had the good fortune to have many happy times with Paul and Terttu in the years that followed. Paul was an outstanding mentor, and somehow things happened when he was around.
3.4 Ruprecht Jaenicke remembers
Ruprecht Jaenicke first met Paul Crutzen at conferences in the early 1970s. They also met at the well-known “Dahlem conferences” in Berlin in the 1980s (Fig. 3). During Paul's time at NCAR (Sect. 2), Ruprecht visited Boulder, and he was invited to Paul's house several times.

Figure 3A photograph of the Dahlem conference in 1982 showing the group on tropospheric aerosols and photochemical reactions. Participants are as indicated below the image. (Picture courtesy of Stu Penkett.)
Paul Crutzen did not have an easy start at the MPIC in Mainz. He had been proposed to the Max-Planck-Gesellschaft (MPG) for the position of director by Christian Junge (Sect. 2). The expert opinions on Paul Crutzen were very positive, but somehow the MPG had problems appointing him – perhaps due to his unusual career (Crutzen, 1996; Müller, 2022; Fishman et al., 2023).
Paul came to an institute (the MPIC) with collegiate management. He became the general director when he joined the institute. As the general director, he was responsible for all of the decisions regarding the institute's personnel. During this time, a severe problem with the MPIC electronic workshop surfaced. The technically excellent head of this workshop also developed instruments and displays for the Porsche company (and he also drove cars from this company). This was the beginning of the digital area in this field. The head of the workshop was eventually dismissed, but there was a long-lasting considerable unrest in the institute. This was a difficult staff issue, but Paul (supported by his co-directors) managed the issue very well for the benefit of all.
Paul wanted to work with PhD students. The chemists of the University of Mainz at that time regarded the trace constituents of the atmosphere of negligible scientific interest. The chemists recommended only PhD students of moderate ability to undertake research on these substance. Christian Junge also suffered from this problem. At the MPIC, there was a chemist Peter Warneck, who was assigned to Paul. Peter Warneck was an outstanding scientist (https://www.mpic.de/4388946/nachruf-prof-warneck, last access: 2 October 2025) who had habilitated at the University of Mainz and was, therefore, permitted to lead doctoral students to a PhD. Peter Warneck and Paul oriented themselves more towards the Institute of Atmospheric Physics, because of the reservations of the chemists at the University of Mainz. Warneck regularly gave lectures on the chemistry of the atmosphere at the University of Mainz. Paul Crutzen also proposed giving lectures, but the dean of the “Fachbereich Physik” (the Institute of Atmospheric Physics was part of that “Fachbereich”) at that time was very formal and strict. Paul Crutzen had a PhD, but his “Filosofie doktor” was not accepted as being equivalent to a habilitation. Therefore, the dean addressed him as “Dr. Paul Crutzen” in letters and not “Prof. Dr. Paul Crutzen”. This was in spite of Paul being appointed to a professorship at the University of Chicago and, later, at University of California at San Diego.
There were a lot of discussions and problems getting Paul appointed as a professor of the Fachbereich/university and, thus, permission to lead students to a PhD at the University of Mainz. Only shortly before Crutzen was honoured with the Nobel Prize was that goal achieved, and Paul became an “Honorarprofessor” at the University of Mainz. Interestingly, because of his many obligations, Paul never gave regular lectures at Mainz; he lectured a few times, and colleagues then stepped in to give lectures.
Paul Crutzen proposed that Ruprecht should write a comprehensive article for the Landolt–Börnstein (Jaenicke, 1988). Years later, Paul also proposed Ruprecht for an oral presentation in Cape Town as part of the process whereby the city of Mainz became a member of the “Great Wine Capitals”. Ruprecht followed both proposals with enthusiasm. They demonstrate the excellent relationship that Ruprecht had with Paul.
3.5 Rich Stolarski remembers
Back in the 1980s, Rich Stolarski, the famous atmospheric modeller from NASA Goddard Space Flight Center, visited Paul in Boulder. Rich enjoyed the interaction with Paul and was a frequent visitor to Mainz in later years. In the 1980s, Paul agreed to let Rich have a copy of the code of his 2D model. Paul generously shared his results and coding. Paul had a printout of the code that he said he needed to go over with Rich so that Rich could understand what Paul had done. They retired to a room where Paul started to go over the code line by line. Rich began to understand why Paul needed to go over the code. Paul did not waste time cleaning up code or improving comments. The code was not easy to read, with seemingly random insertions and cryptic comments like “there seems to be too much diffusion in the upper stratosphere so …...Kzz = 0.5 ⋅ Kzz”. Rich took the punch cards for this code back to Goddard and messed with it for a while, but he eventually gave up because he just could not get anywhere with it. Looking back on this story, Rich remembers the lesson that he learned from that incident: Paul was not a very good programmer. Many wrote better codes. However, Paul was an artist. He wrote codes to test ideas. For him, the code was not the goal – the idea was the goal.
Rich would say, modestly about himself, that he succeeded in writing some imperfect code and even had a few ideas. However, he considered that Paul's genius was not just ideas but, rather, lots of ideas that he could string together into a whole picture. Over the years, Paul went from one picture to another, always with ideas that he knew how to pursue and figure out their implications. He went from mesospheric chemistry to stratospheric chemistry to tropospheric chemistry to aerosols and to the Anthropocene (Sect. 1).
Going back even farther, Rich remembers going to an American Geophysical Union (AGU) meeting in San Francisco where there was a session on the impact of supersonic aircraft on the atmosphere. If he remembers correctly, it was 1970. At that time, Paul was completely unknown. He gave a talk on hydrogen oxide chemistry. Rich remembers him getting up and showing a viewgraph with a lot of chemical equations on it. It came up out of focus, but Paul said that was okay because he was just trying to impress the audience that he had a lot of reactions in his model. Rich liked his humble attitude and desire to determine if any of his work might be of use on this problem.
A few years later (in 1973), Rich Stolarski gave a talk on chlorine chemistry at the IAGA (International Association of Geomagnetism and Aeronomy) meeting in Kyoto, Japan (this talk later led to the well-known paper on the impact of chlorine chemistry on stratospheric ozone; Stolarski and Cicerone, 1974). Ralph Cicerone and Rich Stolarski had gotten onto the idea from, among other things, talking to Don Stedman who declared that “chlorine destroys ozone, everyone knows that!”. After the talk, Paul sat down with Rich in the hall and went through a series of chemical equations that Paul argued might actually lead to an increase in ozone. He later said that he wasted several months trying to prove that chlorine could increase ozone. Looking back on it, Rich thinks that Paul did not waste his time because what he was really doing was being thorough and proving to himself that nobody had made some dumb mistake. In fact, Rich and Ralph (Stolarski and Cicerone, 1974) had made at least one simple omission in their line of arguments that was pointed out by Doug Davis; they had not initially included the formation of HCl. Years later they were doing Monte Carlo uncertainty calculations and it turned out to be within the realm of possibility that chlorine could increase ozone if the reaction rate of OH + HO2 was extremely small. In that case, HOx would have very large concentrations and dominate ozone loss. Chlorine would then catalyse the reaction OH + HO2 via Cl + HO2 ⟶ HCl + O2 and HCl + OH ⟶ Cl + H2O. This, of course, was not so, as the reaction rate for OH + HO2 turned out to be much greater than for this extreme case (as it is known today; Burkholder et al., 2019). Nevertheless, it does show that, in those early days, it was quite reasonable to test the idea that chlorine might cause an increase in ozone. Paul was all about testing ideas, of which he had plenty. Paul and Rich met many times over the years; one occasion was the Quadrennial Ozone Symposium in Kos (Greece) in June 2004 (Fig. 4).
3.6 Rolf Müller remembers
3.6.1 A PhD in Paul's department in Mainz
As discussed above (Sect. 3.4), the relationship between Paul and the University of Mainz was not always easy. However, after receiving the Nobel Prize in Chemistry, both the Max Planck Society and the University of Mainz were very proud of Paul and his scientific achievements. On one occasion, the president of the university brought up Paul's Noble Prize in a speech, mentioning the University of Mainz many times. Paul was listening, while standing next to the “Kanzler” (the financial and administrative head) of the University of Mainz. The latter mentioned to Paul quietly that perhaps there was a bit too much mention of “our university” in the President's speech. Paul replied wisely and humbly (in German2) “that, in any case, what counts is the future (i.e. rather than the past)!”.
Paul was travelling a lot, and people at his institute developed the habit of putting important papers and documents on his chair in the office so that he would find the important material immediately upon his return. However, there were too many important things. Thus, when Paul returned to his office, he frequently found a considerable pile of documents on his chair. The concept did not work; Rolf Müller was present when, after days of absence, Paul returned to his office, moved the entire pile on his chair somewhere else (without paying attention to it) and then sat down in his chair to start a scientific discussion.
On one occasion, Paul asked Rolf and colleagues for the model results (and the plots) for a particular problem on Antarctic ozone chemistry (indeed the plots for the paper by Crutzen et al., 1992). The plots were not ready, and Paul was informed about the circumstances. His reply was that he needed the plots urgently to finish the paper. The response was how could he finish the paper if he had not seen the results and the plots. His response was (with some self-irony) “I know the outcome of the simulations, but I need the plots for the paper”. Paul was always impatient to receive information on new scientific results (and this included being impatient with himself) – this anecdote is an example of his impatience regarding new scientific results.
Paul was always working, but he sometimes put aside a few days of holidays for his family. However, he had an agreement with his wife: he could take one piece work along on his holidays.
3.6.2 A talk in Jülich in February 1996
In early 1995, Rolf Müller moved from Mainz to Jülich, as he had gotten a new job at the Forschungszentrum Jülich. He thought it was a good idea to invite his former boss from Mainz for a seminar; therefore, a plan was made for February 1996 (one already had to plan months in advance to find a time slot with Paul at this time). Seminars were on Thursday afternoons; thus, the seminar was organised for Thursday 15 February 1996. Soon thereafter two things happened: first, Paul Crutzen (along with Mario Molina and Sherry Rowland) was awarded the Nobel Prize for Chemistry in 1995; second, the 15 February 1996 fell on “Fettdonnerstag” or “Wieverfastelovend”, which is the day on which carnival is celebrated in the region's workplaces. (Mainz, the town in which Paul lived at that time, is also a carnival town, but this tradition was less intensively nurtured there.) On “Fettdonnerstag”, people tended to work in the morning, although mostly dressed in carnival costumes, or (definitely after 11:11 in the morning) did not work and were, rather, in “party mode”.
The timing of Paul's visit consequently caused a big commotion at Jülich. The board of directors did not dare to officially welcome the new Nobel Laureate on such a day. However, shifting Paul's visit to another day was not a feasible option either. In the end, it turned out that the big lecture hall was full of people (some in costumes) listening to Paul's talk on the impact of halogens on tropospheric ozone. However, a few minutes after Paul had begun his lecture, the door swung open and a band of “witches” came in with a big pair of scissors. One needs to understand that the “witch” is the traditional women's costume on this day and that women are allowed (only on this day) to cut the tie of a man. However, Paul was not wearing a tie on this occasion. He continued his talk after about 5 min with the words “I hope I will be able to focus on my talk again now”.
Paul J. Crutzen was a brilliant scientist, and whilst he enjoyed his research successes, he was a modest man. Up until the end of his life, he was motivated and fascinated by new challenges and ideas in the atmospheric sciences and the role of humans in determining the behaviour of the Earth system. His focus was not on his past achievements, and Paul was not only a scientist but also a kind-hearted, humorous and pleasant person – something that cannot be said about every great, successful scientist. He was very much interested in the private lives of his colleagues and students, and he always had time for his family. This collection of anecdotes and recollections offers a glimpse into the human side of Paul Crutzen, an aspect that often remains hidden behind his many outstanding scientific accomplishments.
No data sets were used in this article.
All of the co-authors contributed to putting together the material for this paper and to writing the manuscript. LB, JB, JB, RJ and RS contributed the individual memories in Sect. 3. All of the co-authors commented on the draft of the manuscript and contributed to the final version.
At least one of the (co-)authors is a member of the editorial board of Atmospheric Chemistry and Physics. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
First of all, the authors wish to thank Terttu Crutzen for reading and commenting on this manuscript. Further, we thank Paul's daughters, Ilona and Sylvia, for comments and help. We also thank Paul's daughter Ilona and his grandson Jamie Paul for providing pictures from the family archives. The picture from the Dahlem conference in 1982 was provided by Stu Penkett. We thank Christoph Brühl, Astrid Kaltenbach and Sandra Stein for comments. We are also grateful to Reinhard Zellner, who (together with Paul) was active in the “Enquete-Kommission: Vorsorge zum Schutz der Erdatmosphäre” of the German “Bundestag”; he provided some very valuable insight into the scientific driving issues and the results of this commission.
This paper was edited by Kristian Schlegel and reviewed by one anonymous referee.
Benner, S., Lax, G., Crutzen, P. J., Pöschl, U., Lelieveld, J., and Brauch, H. G. (Eds.): Paul J. Crutzen and the Anthropocene: A new epoch in Earth's history, Springer, ISBN: 978-3-030-82202-6, 2021. a
Birks, J. W. and Crutzen, P. J.: Atmospheric effects of a nuclear war, Chem. Brit., 19, 927–930, 1983. a
Burkholder, J. B., Sander, S. P., Abbatt, J. P. D., Barker, J. R., Cappa, C., Crounse, J. D., Dibble, T. S., Huie, R. E., Kolb, C. E., Kurylo, M. J., Orkin, V. L., Percical, C. J., Wilmouth, D. M., and Wine, P. H.: Chemical kinetics and photochemical data for use in atmospheric studies, Evaluation Number 19, JPL Publication 19-5, http://jpldataeval.jpl.nasa.gov (last access: 2 October 2025), 2019. a
Burrows, J., Hölzle, E., Goede, A., Visser, H., and Fricke, W.: SCIAMACHY – scanning imaging absorption spectrometer for atmospheric chartography, Acta Astronaut., 35, 445–451, https://doi.org/10.1016/0094-5765(94)00278-T, 1995. a
Burrows, J. P., Weber, M., Buchwitz, M., Rozanov, V., Ladstätter-Weißenmayer, A., Richter, A., DeBeek, R., Hoogen, R., Bramstedt, K., Eichmann, K.-U., Eisinger, M., and Perner, D.: The Global Ozone Monitoring Experiment GOME: Mission concept and first scientific results, J. Atmos. Sci., 56, 151–175, 1999. a
Chameides, W. and Walker, J. C.: A photochemical theory of tropospheric ozone, J. Geophys. Res., 78, 8751–8760, https://doi.org/10.1029/JC078i036p08751, 1973. a
Crutzen, P. and Ehhalt, D.: Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer, Ambio, 6, 112–117, 1977. a
Crutzen, P. J.: The influence of nitrogen oxides on the atmospheric ozone content, Q. J. Roy. Meteor. Soc., 96, 320–325, 1970. a, b, c
Crutzen, P. J.: SSTs – a threat to the earth's ozone shield, Ambio, 1, 41–51, 1972. a
Crutzen, P. J.: A discussion of the chemistry of some minor constituents in the stratosphere and troposphere, Pure Appl. Geophys., 106, 1385–1399, 1973. a
Crutzen, P. J.: My Life with O3, NOx and other YZOx compounds, Angew. Chem. Intern. Ed., 35, 1747–1871, 1996. a, b, c, d
Crutzen, P. J.: Geology of Mankind, Nature, 415, 23, https://doi.org/10.1038/415023a, 2002. a
Crutzen, P. J.: Albedo enhancements by stratospheric sulfur injections: a contribution to resolve a policy dilemma? An Editorial Essay, Climatic Change, 77, 211–219, 2006. a
Crutzen, P. J. and Andreae, M. O.: Biomass burning in the tropics – Impact on atmospheric chemistry and biogeochemical cycles, Science, 250, 1669–1678, 1990. a
Crutzen, P. J. and Arnold, F.: Nitric acid cloud formation in the cold Antarctic stratosphere: A major cause for the springtime `ozone hole', Nature, 342, 651–655, 1986. a
Crutzen, P. J. and Birks, J. W.: The atmosphere after a nuclear war: Twilight at noon, Ambio, 11, 114–125, 1982. a, b
Crutzen, P. J. and Müller, M. (Eds.): Das Anthropozän, Oekom, ISBN: 978-3-96238-137-0, 2019. a
Crutzen, P. J. and Steffen, W.: How long have we been in the Anthropocene era?, Climatic Change, 61, 251–257, 2003. a
Crutzen, P. J., Heidt, L. E., Krasnec, J. P., Pollock, W. H., and Seiler, W.: Biomass burning as a source of atmospheric gases CO, H2, N2O, NO, CH3Cl and COS, Nature, 282, 253–256, 1979. a
Crutzen, P. J., Müller, R., Brühl, C., and Peter, T.: On the potential importance of the gas phase reaction CH3O2 + ClO → ClOO + CH3O and the heterogeneous reaction HOCl + HCl → H2O + Cl2 in “ozone hole” chemistry, Geophys. Res. Lett., 19, 1113–1116, https://doi.org/10.1029/92GL01172, 1992. a
Crutzen, P. J., Grooß, J.-U., Brühl, C., Müller, R., and Russell III, J. M.: A Reevaluation of the ozone budget with HALOE UARS data: No evidence for the ozone deficit, Science, 268, 705–708, 1995. a
Dickman, S.: Atmosphere may rise to the top of the West German agenda, Nature, 334, 459, https://doi.org/10.1038/334459a0, 1988. a
Ervens, B., Carslaw, K. S., Koop, T., and Pöschl, U.: Opinion: Improved scientific discourse and quality assurance by interactive open access publishing with community-based multi-stage open peer review in an open science landscape, EGUsphere [preprint], https://doi.org/10.5194/egusphere-2025-419, 2025. a, b
Farman, J. C., Gardiner, B. G., and Shanklin, J. D.: Large losses of total ozone in Antarctica reveal seasonal ClONOx interaction, Nature, 315, 207–210, https://doi.org/10.1038/315207a0, 1985. a
Fishman, J. and Crutzen, P.: The origin of ozone in the troposphere, Nature, 274, 855–858, https://doi.org/10.1038/274855a0, 1978. a
Fishman, J., Ramanathan, V., Crutzen, P. J., and Liu, S. C.: Tropospheric ozone and climate, Nature, 282, 818–820, 1979a. a
Fishman, J., Solomon, S., and Crutzen, P. J.: Observational and theoretical evidence in support of a significant in situ photochemical source of tropospheric ozone, Tellus, 31, 432–446, 1979b. a
Fishman, J., Birks, J. W., Graedel, T. E., Steffen, W., Burrows, J. P., Howard, C. J., and Wayne, R. P.: A Tribute to Paul Crutzen (1933–2021): The pioneering atmospheric chemist who provided new insight into the concept of climate change, B. Am. Meteorol. Soc., 104, E77–E95, https://doi.org/10.1175/BAMS-D-21-0311.1, 2023. a, b, c, d, e, f, g
Harris, N. R. P.: Frank Sherwood `Sherry' Rowland, Biographical Memoirs of Fellows of the Royal Society, 68, 371–383, https://doi.org/10.1098/rsbm.2019.0032, 2020. a
Jaenicke, R.: Numerical Data and Functional Relations in Science and Technology, New Series, Group V, Vol. 4, Meteorology, Subvolume b, Physical and Chemical Properties of the Air, Aerosol physics and chemistry, Chap. 9, Landolt-Börnstein, ISBN 978-3540176039, 1988. a
Johnston, H.: Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust, Science, 173, 517–522, 1971. a
Kuttippurath, J., Lefèvre, F., Pommereau, J.-P., Roscoe, H. K., Goutail, F., Pazmiño, A., and Shanklin, J. D.: Antarctic ozone loss in 1979–2010: first sign of ozone recovery, Atmos. Chem. Phys., 13, 1625–1635, https://doi.org/10.5194/acp-13-1625-2013, 2013. a
Lax, G.: From atmospheric chemistry to earth system science, GNT Verlag, Diepholz-Berlin, ISBN 978-3-86225-112-4, 2018. a, b
Lelieveld, J.: Paul J. Crutzen (1933–2021), Nature, 591, 29, https://doi.org/10.1038/d41586-021-00479-0, 2021. a
Lelieveld, J., Crutzen, P. J., Ramanathan, V., Andreae, M. O., Brenninkmeijer, C. A. M., Campos, T., Cass, G. R., Dickerson, R. R., Fischer, H., de Gouw, J. A., Hansel, A., Jefferson, A., Kley, D., de Laat, A. T. J., Lal, S., Lawrence, M. G., Lobert, J. M., Mayol-Bracero, O. L., Mitra, A. P., Novakov, T., Oltmans, S. J., Prather, K. A., Reiner, T., Rodhe, H., Scheeren, H. A., Sikka, D., and Williams, J.: The Indian Ocean Experiment: Widespread Air Pollution from South to Southeast Asia, Science, 291, 1031–1036, 2001. a
Möllers, N., Schwägerl, C., and Trischler, H. (Eds.): Willkommen im Anthropozän, Deutsches Museum Verlag, ISBN 978-3-940396-48-8, 2015. a
Moortgat, G. K., Burrows, J. P., and Lammel, G.: Paul J. Crutzen (1933–2021), Nachr. Chem., 69, 83, https://doi.org/10.1002/nadc.20214109390, 2021. a
Müller, R.: Paul Jozef Crutzen. 3 December 1933–28 January 2021, Biographical Memoirs of Fellows of the Royal Society, 73, 127–156, https://doi.org/10.1098/rsbm.2022.0011, 2022. a, b, c, d, e, f, g, h, i
Müller, R., Pöschl, U., Koop, T., Peter, T., and Carslaw, K.: Paul J. Crutzen – a pioneer in Earth system science and a founding member of the journal Atmospheric Chemistry and Physics, Atmos. Chem. Phys., 23, 15445–15453, https://doi.org/10.5194/acp-23-15445-2023, 2023. a, b, c, d, e
Perner, D. and Platt, U.: Detection of Nitrous Acid in the Atmosphere by Differential Optical Absorption, Geophys. Res. Lett., 6, 917–920, 1979. a
Pöschl, U.: Multi-Stage Open Peer Review: Scientific Evaluation Integrating the Strengths of Traditional Peer Review with the Virtues of Transparency and Self-Regulation, Front. Comput. Neurosc., 6, 33, https://doi.org/10.3389/fncom.2012.00033, 2012. a
Ramanathan, V., Crutzen, P. J., Kiehl, J. T., and Rosenfeld, D.: Aerosols, Climate, and the Hydrological Cycle, Science, 294, 2119–2124, 2001. a
Robock, A.: Snow and ice feedbacks prolong effects of nuclear winter, Nature, 310, 667–670, 1984. a
Robock, A., Xia, L., Harrison, C. S., Coupe, J., Toon, O. B., and Bardeen, C. G.: Opinion: How fear of nuclear winter has helped save the world, so far, Atmos. Chem. Phys., 23, 6691–6701, https://doi.org/10.5194/acp-23-6691-2023, 2023. a, b
Rodhe, H.: Paul Crutzen 1933–2021, Royal Swedish Academy of Sciences, Stockholm, https://www.su.se/cmlink/stockholmsuniversitet-naturvetenskapliga-fakulteten/meteorologiska-institutionennod/meteorologiska-institutionen/till-minne-paul-crutzen-1.548258 (last access: 2 October 2025), 2021 (in Swedish). a
Russell III, J. M., Gordley, L. L., Park, J. H., Drayson, S. R., Hesketh, W. D., Cicerone, R. J., Tuck, A. F., Frederick, J. E., Harries, J. E., and Crutzen, P. J.: The Halogen Occultation Experiment, J. Geophys. Res., 98, 10777–10797, https://doi.org/10.1029/93JD00799, 1993. a
Schmidbauer, B. (Ed.): Enquete-Kommission des Dt. Bundestags: “Vorsorge zum Schutz der Erdatmosphäre”, Economica Verlag, 3. Auflage, ISBN: 3-924521-61-1, 1990. a, b, c
Schütz, K., Junge, C., Beck, R., and Albrecht, B.: Studies of atmospheric N2O, J. Geophys. Res., 75, 2230–2246, https://doi.org/10.1029/JC075i012p02230, 1970. a
Solomon, S.: Paul J. Crutzen (1933–2021), Science, 371, 892, https://doi.org/10.1126/science.abh0217, 2021. a
Solomon, S. and Crutzen, P. J.: Analysis of the August 1972 Solar Proton Event including chlorine chemistry, J. Geophys. Res., 86, 1140–1146, https://doi.org/10.1029/JC086iC02p01140, 1981. a
Solomon, S., Ivy, D. J., Kinnison, D., Mills, M. J., Neely, R. R., and Schmidt, A.: Emergence of healing in the Antarctic ozone layer, Science, 353, 269–274, https://doi.org/10.1126/science.aae0061, 2016. a
Stolarski, R. S. and Cicerone, R. J.: Stratospheric chlorine: A possible sink of ozone, Canad. J. Chem., 52, 1610–1615, 1974. a, b
Turco, R. P., Toon, O. B., Ackerman, T. P., Pollack, J. B., and Sagan, C.: Nuclear winter: Global consequences of multiple nuclear explosions, Science, 222, 1283–1292, https://doi.org/10.1126/science.222.4630.1283, 1983. a
Visioni, D., Kravitz, B., Robock, A., Tilmes, S., Haywood, J., Boucher, O., Lawrence, M., Irvine, P., Niemeier, U., Xia, L., Chiodo, G., Lennard, C., Watanabe, S., Moore, J. C., and Muri, H.: Opinion: The scientific and community-building roles of the Geoengineering Model Intercomparison Project (GeoMIP) – past, present, and future, Atmos. Chem. Phys., 23, 5149–5176, https://doi.org/10.5194/acp-23-5149-2023, 2023. a
WMO: Scientific assessment of ozone depletion: 2022, GAW Report No. 278, Geneva, Switzerland, 2022. a, b, c
Zalasiewicz, J., Waters, C., and Steffen, W.: Remembering the extraordinary scientist Paul Crutzen (1933–2021), Sci. Am., https://www.scientificamerican.com/article/remembering-the-extraordinary-scientist-paul-crutzen-1933-2021/ (last access: 2 October 2025), 5 February 2021. a
Zetzsch, C.: Paul Crutzen: 1933–2021, Toxicol. Environ. Chem., 103, 238–243, https://doi.org/10.1080/02772248.2021.1941022, 2021. a





